|
|
For More Information
- HER2 Support Group
- Breast Cancer Treatment
- Guide For Women
- Metastatic Breast Cancer
- Staging
- Sources of Support
- Screening
- Nutrition & Physical Activity
- NCI Publications
- Follow-Up Care
- Complimentary & Alternative Medicine
- Breast Reconstruction
- Risk Factors
- Cancer & Cancer Pathways
- MD Anderson Cancer Handbook
- Your Guide to Cancer Care
- How To Handle The Fear of Breast Cancer
| HER2 Breast Cancer - Page 2 |
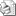 |
Page 2 of 2
Boxed WARNINGS and Additional Important Safety Information Herceptin administration can result in sub-clinical and clinical cardiac failure manifesting as congestive heart failure (CHF) and decreased left ventricular ejection fraction (LVEF). The incidence and severity of left ventricular cardiac dysfunction was highest in patients who received Herceptin concurrently with anthracycline-containing chemotherapy regimens. Discontinue Herceptin treatment in patients receiving adjuvant therapy and strongly consider discontinuation of Herceptin in patients with metastatic breast cancer who develop a clinically significant decrease in left ventricular function. Patients should undergo monitoring for decreased left ventricular function before Herceptin treatment, and frequently during and after Herceptin treatment. More frequent monitoring should be employed if Herceptin is withheld in patients who develop significant left ventricular cardiac dysfunction. In one adjuvant clinical trial, cardiac ischemia or infarction occurred in the Herceptin containing regimens. Serious infusion reactions and pulmonary toxicity have occurred; fatal infusion reactions have been reported. In most cases, symptoms occurred during or within 24 hours of administration of Herceptin. Herceptin infusion should be interrupted for patients experiencing dyspnea or clinically significant hypotension. Patients should be monitored until signs and symptoms completely resolve. Discontinue Herceptin for infusion reactions manifesting as anaphylaxis, angioedema, interstitial pneumonitis, or acute respiratory distress syndrome. Exacerbation of chemotherapy-induced neutropenia has also occurred. Herceptin can cause oligohydramnios and fetal harm when administered to a pregnant woman. The most common adverse reactions associated with Herceptin use were fever, nausea, vomiting, infusion reactions, diarrhea, infections, increased cough, headache, fatigue, dyspnea, rash, neutropenia, anemia, and myalgia. Mechanism of Action Herceptin is a humanized monoclonal antibody (also called a biologic therapy). Antibodies are part of the body's normal defense against bacteria, viruses and abnormal cells such as cancer cells. Therapeutic monoclonal antibodies are created and produced in a laboratory through a complex and resource-intensive process. Their name comes from the fact that they are produced from a single cell.1 Based on preclinical studies, Herceptin works on both the extracellular and the intracellular domains of the HER2 receptor.2-5
Mechanism of action of herceptin3-5,8-10
1 Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci. 1992;89:4285-4289. 2 Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin). Semin Oncol. 1999;26:60-70. 3 Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidyli-nositol-3 kinase and Akt is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Research. 2002;62:4132-4141. 4 Arnould L, Gelly M, Penault-Llorca F. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259-267. 5 Bianco AR. Targeting c-erb2 and other receptors of the c-erB family: rationale and clinical applications. J Chemother. 2004;16:52-54 . 6 Pegram MD, Konecny GE, O'Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Nat Cancer Inst. 2004;96:739-749. 7 Baselga J, Norton L, Albanell J, Kim Y-M, Mendelsohn J. Recombinant humanized anti-HER2 antibody (HerceptinTM) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58:2825-2831. 8 Lewis GD, Figari I, Fendly B. Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunol Immunother. 1993;37:255-263. 9 Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61:1-13. 10 Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19:6102-6114.
|
||||||
| Last Updated on Thursday, 18 March 2010 07:02 |

 HER2 Breast Cancer
HER2 Breast Cancer