 |
|
 07-09-2007, 10:05 AM
07-09-2007, 10:05 AM
|
#1
|
|
Senior Member
Join Date: Mar 2006
Posts: 4,778
|
here we go again: How inexact ER status testing is!!!
Not to worry you...these papers are useful. At the most recent bc conference I attended they even discussed changing the method by which ER is tested to make it more exact. How long that might take is another matter...Some of the fault may lie in the testing (and testers) but the tumor itself may have some areas with more and some with less ER
ABSTRACT: Quantitative analysis of estrogen receptor heterogeneity in breast cancer [Laboratory Investigation]
Immunohistochemical analyses (IHC) of biomarkers are extensively used for tumor characterization and as prognostic and predictive measures. The current standard of single slide analysis assumes that one 5 ?M section is representative of the entire tumor. We used our automated image analysis technology (AQUA) using a modified IHC technique with fluorophores to compare estrogen receptor (ER) expression in multiple blocks/slides from cases of primary breast cancer with the objective of quantifying tumor heterogeneity within sections and between blocks. To normalize our ER scores and allow slide-to-slide comparisons, 0.6 ?m histospots of representative breast cancer cases with known ER scores were assembled into a 'gold standard array' (GSA) and placed adjacently to each whole section. Overall, there was excellent correlation between AQUA scores and the pathologist's scores and reproducibility of GSA scores (mean linear regression R value 0.8903). Twenty-nine slides from 11 surgical cases were then analyzed totaling over 2000 AQUA images. Using standard binary assignments of AQUA (>10) and pathologist's (>10%) scores as being positive, there was fair concordancy between AQUA and pathologist scores (73%) and between slides from different blocks from the same cases (75%). However using continuous AQUA scores, agreement between AQUA and pathologist was far lower and between slides from different blocks from the same cases only 19%. Within individual slides there was also significant heterogeneity in a scattered pattern, most notably for slides with the highest AQUA scores. In sum, using a quantitative measure of ER expression, significant block-to-block heterogeneity was found in 81% of cases. These results most likely reflect both laboratory-based variability due to lack of standardization of immunohistochemistry and true biological heterogeneity. It is also likely to be dependent on the biomarker analyzed and suggests further studies should be carried out to determine how these findings may affect clinical decision-making processes.
|

|

|
 07-09-2007, 02:41 PM
07-09-2007, 02:41 PM
|
#2
|
|
Senior Member
Join Date: Jun 2006
Location: Bradenton,FL
Posts: 977
|
Oh My Goodness Lani, I NEVER thought to question my ER status, nor the method that was used to test for it! I DID make MDAnderson repeat my Her neu test using FISH (IHC used initially).
You are amazing! Thank you for all you bring to our attention!
Marcia
Last edited by Soccermom; 07-09-2007 at 02:42 PM..
Reason: misspelling
|

|

|
 07-09-2007, 06:48 PM
07-09-2007, 06:48 PM
|
#3
|
|
Senior Member
Join Date: Mar 2006
Posts: 4,778
|
Marcia
In case you didn't catch it, within the last month I posted a study out of the UK showing that the use of methylene blue dye by the surgeon to identify the
sentinel node and/or the track of the localization needle for biopsy alters the result of the most common method of ER testing such that there appears to be less ER than there truly are. Utilize search and look up methylene blue.
Other more expensive dyes did not, but methylene blue was used overwhelmingly in the US for these purposes.
|

|

|
 07-09-2007, 07:49 PM
07-09-2007, 07:49 PM
|
#4
|
|
Senior Member
Join Date: Jun 2006
Location: Bradenton,FL
Posts: 977
|
Lani...I must have missed that one! Which may be a good thing (for me) as I tend to get soooo worked up over things I cannot change.
Right now I am preparing to do battle with my Insurer so am preparing my plan of attack...
Michael Moore "LOOK OUT"!
Thanks again Lani!
Marcia
Thank you again!
Marcia
|

|

|
 07-09-2007, 07:55 PM
07-09-2007, 07:55 PM
|
#5
|
|
Senior Member
Join Date: Aug 2006
Posts: 3,380
|
Lani,
Thanks for posting this report. I was reading an article last year on the possibility for some ER+ women with small cancers not to be routinely rx with chemo, and one of the doctors interviewed said part of the reluctance to come out with such a guideline had to do with the inexactitude, for want of a better word, of ER testing - that it vaired more from lab to lab than Her2 testing, and that it was the big problem no one talked about.
I have also read that there is a difference in the intensity of the ER staining, which provides additional info to the sheer number of the cells that stain for positive. Do you have any info on that?
Thanks,
Hopeful
|

|

|
 07-10-2007, 12:02 AM
07-10-2007, 12:02 AM
|
#6
|
|
Senior Member
Join Date: Mar 2006
Posts: 4,778
|
Hopeful--here is a quick article will post others if I have a chance
J Natl Cancer Inst. 2006 Nov 1;98(21):1571-81. Links
Re-evaluating adjuvant breast cancer trials: assessing hormone receptor status by immunohistochemical versus extraction assays.
Regan MM, Viale G, Mastropasqua MG, Maiorano E, Golouh R, Carbone A, Brown B, Suurküla M, Langman G, Mazzucchelli L, Braye S, Grigolato P, Gelber RD, Castiglione-Gertsch M, Price KN, Coates AS, Goldhirsch A, Gusterson B; International Breast Cancer Study Group.
IBCSG Statistical Center, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, 44 Binney St., Boston, MA 02115, USA. mregan@jimmy.harvard.edu
BACKGROUND: Tumor levels of steroid hormone receptors, a factor used to select adjuvant treatment for early-stage breast cancer, are currently determined with immunohistochemical assays. These assays have a discordance of 10%-30% with previously used extraction assays. We assessed the concordance and predictive value of hormone receptor status as determined by immunohistochemical and extraction assays on specimens from International Breast Cancer Study Group Trials VIII and IX. These trials predominantly used extraction assays and compared adjuvant chemoendocrine therapy with endocrine therapy alone among pre- and postmenopausal patients with lymph node-negative breast cancer. Trial conclusions were that combination therapy provided a benefit to pre- and postmenopausal patients with estrogen receptor (ER)-negative tumors but not to ER-positive postmenopausal patients. ER-positive premenopausal patients required further study. METHODS: Tumor specimens from 571 premenopausal and 976 postmenopausal patients on which extraction assays had determined ER and progesterone receptor (PgR) levels before randomization from October 1, 1988, through October 1, 1999, were re-evaluated with an immunohistochemical assay in a central pathology laboratory. The endpoint was disease-free survival. Hazard ratios of recurrence or death for treatment comparisons were estimated with Cox proportional hazards regression models, and discriminatory ability was evaluated with the c index. All statistical tests were two-sided. RESULTS: Concordance of hormone receptor status determined by both assays ranged from 74% (kappa = 0.48) for PgR among postmenopausal patients to 88% (kappa = 0.66) for ER in postmenopausal patients. Hazard ratio estimates were similar for the association between disease-free survival and ER status (among all patients) or PgR status (among postmenopausal patients) as determined by the two methods. However, among premenopausal patients treated with endocrine therapy alone, the discriminatory ability of PgR status as determined by immunohistochemical assay was statistically significantly better (c index = 0.60 versus 0.51; P = .003) than that determined by extraction assay, and so immunohistochemically determined PgR status could predict disease-free survival. CONCLUSIONS: Trial conclusions in which ER status (for all patients) or PgR status (for postmenopausal patients) was determined by immunohistochemical assay supported those determined by extraction assays. However, among premenopausal patients, trial conclusions drawn from PgR status differed--immunohistochemically determined PgR status could predict response to endocrine therapy, unlike that determined by the extraction assay.
PMID: 17077359 [PubMed - indexed for MEDLINE] |

|

|
 07-10-2007, 12:09 AM
07-10-2007, 12:09 AM
|
#7
|
|
Senior Member
Join Date: Mar 2006
Posts: 4,778
|
more......
: Am J Clin Pathol. 2007 Mar;127(3):356-65. Links
Comparison of evaluations for hormone receptors in breast carcinoma using two manual and three automated immunohistochemical assays.
Arihiro K, Umemura S, Kurosumi M, Moriya T, Oyama T, Yamashita H, Umekita Y, Komoike Y, Shimizu C, Fukushima H, Kajiwara H, Akiyama F.
Department of Anatomical Pathology, Hiroshima University Hospital, Hiroshima, Japan.
The aims of this study were to compare the quality of immunohistochemical assays of estrogen receptor (ER) and progesterone receptor (PR) and to compare the intermethod variability of assays from different manufacturers. immunohistochemical staining was entrusted to the following laboratories in Japan: Kyowa Medex, dealing with the products of BioGenex (Mishima, Shizuoka), DAKO Japan (Kyoto) and Ventana Japan (Yokohama). All slides were semiquantitatively evaluated according to the Allred score. Intermethod variability showed fair to moderate multirater kappa values for ER and PR (for total score, ER, kappa = 0.34; PR, kappa = 0.45). Another scoring system was also applied in which, irrespective of the intensity of nuclear staining, the proportion of cells stained in each specimen was recorded as 0; less than 1%; 1% or more and less than 10%; or 10% or more. Intermethod variability showed substantial multirater kappa values for ER and PR (according to percentage of positive cells, ER, kappa = 0.67; PR, kappa = 0.72). Concerning intermethod consistency, the scoring system based on the percentage of positive cells was advantageous over other scoring systems.
PMID: 17276950 [PubMed - indexed for MEDLINE]
Related Links
Quality assurance for detection of estrogen and progesterone receptors by immunohistochemistry in Austrian pathology laboratories. [Virchows Arch. 2002]
The effects of fixation, processing and evaluation criteria on immunohistochemical detection of hormone receptors in breast cancer. [Breast Cancer. 2007]
Interobserver reproducibility of immunocytochemical estrogen- and progesterone receptor status assessment in breast cancer. [Anticancer Res. 1996]
Immunohistochemical demonstration of oestrogen and progesterone receptors: correlation of standards achieved on in house tumours with that achieved on external quality assessment material in over 150 laboratories from 26 countries. [J Clin Pathol. 2000]
Simultaneous immunohistochemical and biochemical hormone receptor assessment in breast cancer provides complementary prognostic information. [Anticancer Res. 1997]
See all Related Articles...
|

|

|
 07-10-2007, 12:12 AM
07-10-2007, 12:12 AM
|
#8
|
|
Senior Member
Join Date: Mar 2006
Posts: 4,778
|
and more....
Rhodes A, Jasani B, Balaton AJ, Miller KD.
Related Articles, Links
Immunohistochemical demonstration of oestrogen and progesterone receptors: correlation of standards achieved on in house tumours with that achieved on external quality assessment material in over 150 laboratories from 26 countries.
J Clin Pathol. 2000 Apr;53(4):292-301.
PMID: 10823126 [PubMed - indexed for MEDLINE]
6:
Biesterfeld S, Schroder W, Steinhagen G, Koch R, Veuskens U, Schmitz FJ, Handt S, Bocking A.
Related Articles, Links
Simultaneous immunohistochemical and biochemical hormone receptor assessment in breast cancer provides complementary prognostic information.
Anticancer Res. 1997 Nov-Dec;17(6D):4723-9.
PMID: 9494596 [PubMed - indexed for MEDLINE]
7:
Ogawa Y, Moriya T, Kato Y, Oguma M, Ikeda K, Takashima T, Nakata B, Ishikawa T, Hirakawa K.
Related Articles, Links
Immunohistochemical assessment for estrogen receptor and progesterone receptor status in breast cancer: analysis for a cut-off point as the predictor for endocrine therapy.
Breast Cancer. 2004;11(3):267-75.
PMID: 15550845 [PubMed - indexed for MEDLINE]
8:
Fisher ER, Anderson S, Dean S, Dabbs D, Fisher B, Siderits R, Pritchard J, Pereira T, Geyer C, Wolmark N.
Related Articles, Links
Solving the dilemma of the immunohistochemical and other methods used for scoring estrogen receptor and progesterone receptor in patients with invasive breast carcinoma.
Cancer. 2005 Jan 1;103(1):164-73.
PMID: 15565575 [PubMed - indexed for MEDLINE]
9:
Helin HJ, Helle MJ, Helin ML, Isola JJ.
Related Articles, Links
Immunocytochemical detection of estrogen and progesterone receptors in 124 human breast cancers.
Am J Clin Pathol. 1988 Aug;90(2):137-42.
PMID: 2456008 [PubMed - indexed for MEDLINE]
10:
Krishnamurthy S, Dimashkieh H, Patel S, Sneige N.
Related Articles, Links
Immunocytochemical evaluation of estrogen receptor on archival Papanicolaou-stained fine-needle aspirate smears.
Diagn Cytopathol. 2003 Dec;29(6):309-14.
PMID: 14648786 [PubMed - indexed for MEDLINE]
11:
Nichols GE, Frierson HF Jr, Boyd JC, Hanigan MH.
Related Articles, Links
Automated immunohistochemical assay for estrogen receptor status in breast cancer using monoclonal antibody CC4-5 on the Ventana ES.
Am J Clin Pathol. 1996 Sep;106(3):332-8.
PMID: 8816590 [PubMed - indexed for MEDLINE]
12:
Tabbara SO, Sidawy MK, Frost AR, Brosky KR, Coles V, Hecht S, Radcliffe G, Sherman ME.
Related Articles, Links
The stability of estrogen and progesterone receptor expression on breast carcinoma cells stored as PreservCyt suspensions and as ThinPrep slides.
Cancer. 1998 Dec 25;84(6):355-60.
PMID: 9915137 [PubMed - indexed for MEDLINE]
13:
Wilbur DC, Willis J, Mooney RA, Fallon MA, Moynes R, di Sant'Agnese PA.
Related Articles, Links
Estrogen and progesterone receptor detection in archival formalin-fixed, paraffin-embedded tissue from breast carcinoma: a comparison of immunohistochemistry with the dextran-coated charcoal assay.
Mod Pathol. 1992 Jan;5(1):79-84.
PMID: 1371874 [PubMed - indexed for MEDLINE]
14:
Maiorana A, Cavallari V, Bagni A, Ussia F, Maiorana MC, Fano RA.
Related Articles, Links
Nuclear areas in breast cancer: relationship with estrogen and progesterone receptor expression.
Anal Cell Pathol. 1996 Aug;11(3):199-209.
PMID: 8888955 [PubMed - indexed for MEDLINE]
15:
Chebil G, Bendahl PO, Ferno M; South Sweden Breast Cancer Group; North Sweden Breast Cancer Group.
Related Articles, Links
Estrogen and progesterone receptor assay in paraffin-embedded breast cancer--reproducibility of assessment.
Acta Oncol. 2003;42(1):43-7.
PMID: 12665330 [PubMed - indexed for MEDLINE]
16:
Golouh R, Vrhovec I, Bracko M, Frkovic-Grazio S.
Related Articles, Links
Comparison of standardized immunohistochemical and biochemical assays for estrogen and progesterone receptors in breast carcinoma.
Pathol Res Pract. 1997;193(8):543-9.
PMID: 9406247 [PubMed - indexed for MEDLINE]
17:
Biesterfeld S, Kraus HL, Reineke T, Muys L, Mihalcea AM, Rudlowski C.
Related Articles, Links
Analysis of the reliability of manual and automated immunohistochemical staining procedures. A pilot study.
Anal Quant Cytol Histol. 2003 Apr;25(2):90-6.
PMID: 12746978 [PubMed - indexed for MEDLINE]
18:
Keshgegian AA.
Related Articles, Links
Biochemically estrogen receptor-negative, progesterone receptor-positive breast carcinoma. Immunocytochemical hormone receptors and prognostic factors.
Arch Pathol Lab Med. 1994 Mar;118(3):240-4.
PMID: 8135626 [PubMed - indexed for MEDLINE]
19:
Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR.
Related Articles, Links
Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers.
Am J Clin Pathol. 2005 Jan;123(1):21-7.
PMID: 15762276 [PubMed - indexed for MEDLINE]
20:
Leers MP, Hoop JG, van Beers M, van Rodijnen N, Pannebakker M, Nap M.
Related Articles, Links
Determination of threshold values for determining the size of the fraction of steroid hormone receptor-positive tumor cells in paraffin-embedded breast carcinomas.
Cytometry B Clin Cytom. 2005 Mar;64(1):43-52.
PMID: 15668953 [PubMed - indexed for MEDLINE]
Items 1 - 20 of 105
|

|

|
 07-10-2007, 05:10 AM
07-10-2007, 05:10 AM
|
#9
|
|
Senior Member
Join Date: Aug 2006
Posts: 3,380
|
Lani, you are amazing! Thanks so much. 
Hopeful |

|

|
 07-10-2007, 07:51 AM
07-10-2007, 07:51 AM
|
#10
|
|
Senior Member
Join Date: Apr 2007
Location: LA LA Land
Posts: 1,607
|
er/pr negative to positive? her2+++ to ---???
Hi Lani,
Based upon feedback from this site, I asked my onc yesterday if we shouldn't do a biopsy (maybe from lung mets) to see what this cancer is. I asked if er/pr negative can "turn" positive - he said NO. I asked if her2+++ can turn negative - he said NO. The last time they removed something from me, it was the fall of 2005, a local tiny recurrance right at scarline.
He said he "knew" what my cancer was and there was absolutely no reason to do a biopsy. He said a lung mets biopsy wouldn't tell him about the other areas.
Sigh...after reading your posts it's hard not to wonder and worry.
BTW, what is your DX and treatment?
Thanks for all this valuable info. Blessings.
Flori
__________________
1996 cancer WTF?! 1.3 cm lumpectomy Er/Pr neg. Her2+ (20nodes NEGATIVE) did CMF + rads. NED.
2002 recurrence. Bilateral mastectomy w/TFL autologous recon. Then ACx2. Skin lymphatic rash. Taxotere w/Herceptin x4. Herceptin/Xeloda. Finally stops spreading.
2003 - Back to surgery, remove skin mets, and will have surgery one week later when pathology can confirm margins.
‘03 latisimus dorsi flap to remove skin mets. CLEAN MARGINS. Continue single agent Herceptin thru 4/04. NED.
‘04 '05 & 06 tiny recurrences - scar line. surgery to cut out. NED each time.
1/2006 Rads again, to scar line. NED.
3/07 Heartbreaking news - mets! lungs.sternum. Try Tykerb/Xeloda. Tykerb/Carbo/Gemzar. Switch Oncs.
12/07 Herceptin.Tykerb. Markers go stable.
2/8/08 gamma knife 13mm stupid brain met.
3/08 Herceptin/tykerb/avastin/zometa.
3/09 brain NED. Lungs STABLE.
4/09 attack sternum (10 daysPHOTONS.5 days ELECTRONS)
9/09 MARKERS normal!
3/10 PET/CT=manubrium intensely metabolically active but stable. NEDhead.
Wash out 5/10 for tdm1 but 6/10 CT STABLE, PET improving. Markers normal. Brain NED. Resume just Herceptin plus ZOMETA
Dec 2010 Brain NED, lungs/sternum stable. markers normal.
MAR 2011 stop Herceptin/allergy! Go back on Tykerb and switch to Xgeva.
May-Aug 2011 Tykerb Herceptin Xgeva.
Sept 2011 Tykerb, Herceptin, Zometa, Avastin.
April 2012 sketchy drug trial in NYC. 6 weeks later I’m NED!
OCT 2012 PET/CT shows a bunch of freakin’ progression. Back to LA and Herceptin.avastin.zometa.
12/20/12 add in PERJETA!
March 2013 – 5 YEARS POST continue HAPZ
APRIL 2013 - 6 yrs stage 4. "FAILED" PETscan on 4/2/13
May 2013: rePetted - improvement in lungs, left adrenal stable, right 6th rib inactive, (must be PERJETA avastin) sternum and L1 fruckin'worsen. Drop zometa. ADD Xgeva. Doc says get rads consultant for L1 and possible biopsy of L1. I say, no thanks, doc. Lets see what xgeva brings to the table first. It's summer.
June-August 2013HAPX Herceptin Avastin Perjeta xgeva.
Sept - now - on chemo hold for calming tummy we hope. Markers stable for 2 months.
Nov 2013 - Herceptin-Perjeta-Avastin-Xgeva (collageneous colitis, which explains tummy probs, added Entocort)
December '13 BRAIN MRI ned in da head.
Jan 2014: CONTINUING on HAPX…
FEB 2014 PetCT clinical “impression”: 1. newbie nodule - SUV 1.5 right apical nodule, mildly hypermetabolic “suggestive” of worsening neoplastic lesion. 2. moderate worsening of the sternum – SUV 5.6 from 3.8
3. increasing sclerosis & decreasing activity of L1 met “suggests” mild healing. (SUV 9.4 v 12.1 in May ‘13)
4. scattered lung nodules, up to 5mm in size = stable, no increased activity
5. other small scattered sclerotic lesions, one in right iliac and one in thoracic vertebral body similar in appearance to L1 without PET activity and not clearly pathologic
APRIL 2014 - 6 YRS POST GAMMA ZAP, 7 YRS MBC & 18 YEARS FROM ORIGINAL DX!
October 2014: hold avastin, continue HPX
Feb 2015 Cancer you lost. NEDHEAD 7 years post gamma zap miracle, 8 years ST4, +19 yrs original diagnosis.
Continue HPX. Adding back Avastin
Nov 2015 pet/ct is mixed result. L1 SUV is worse. Continue Herceptin/avastin/xgeva. Might revisit Perjeta for L1. Meantime going for rads consult for L1
December 2015 - brain stable. Continue Herceptin, Perjeta, Avastin and xgeva.
Jan 2016: 5 days, 20 grays, Rads to L1 and continue on HAPX. I’m trying to "save" TDM1 for next line. Hope the rads work to quiet L1. Sciatic pain extraordinaire :((
Markers drop post rads.
2/24/16 HAP plus X - markers are down
SCIATIC PAIN DEAL BREAKER.
3/23/16 Laminectomy w/coflex implant L4/5. NO MORE SCIATIC PAIN!!! Healing.
APRIL 2016 - 9 YRS MBC
July 2016 - continue HAP plus Xgeva.
DEC 2016 - PETCT: mets to sternum, lungs, L1 still about the same in size and PET activity. Markers not bad. Not making changes if I don't need to. Herceptin/Perjeta/Avastin/Xgeva
APRIL 2017 10 YEARS MBC
December 2017 - Progression - gonna switch it up
FEB 2018 - Kadcyla 3 cycles ---->progression :(
MAY30th - bronchoscopy, w/foundation1 - her2 enriched
Aug 27, 2018 - start clinical trial ZW25
JAN 2019 - ZW25 seems to be keeping me stable
APRIL 2019 - ONE DOZEN YEARS LIVING METASTATIC
MAY 2019 - progression back on herceptin add xeloda
JUNE 2019 - "6 mos average survival" LMD & CNS new single brain met - one zap during 5 days true beam SBRT to cord met
10/30/19 - stable brain and cord. progression lungs and bones. washing out. applying for ds8201a w nivolumab. hope they take me.
12/27/19 - begin ds8401a w nivolumab. after 2nd cycle nodes melt away. after 3rd cycle chest scan shows Improvement, brain MRI shows improvement, resolved areas & nothing new. switch to plain ENHERTU. after 4th cycle, PETscan shows mostly resolved or improved results. Markers near normal. I'm stunned but grateful.
10/26/20 - June 2021 Tucatinib/xeloda/herceptin - stable ish.
|

|

|
 07-10-2007, 08:34 AM
07-10-2007, 08:34 AM
|
#11
|
|
Senior Member
Join Date: May 2007
Location: DFW area (TX)
Posts: 431
|
Lani...
I think I may have posted these elsewhere but haven't received opinions yet:
1) Is it possible that ER+/HER2+ tumors "need" chemo or react to chemo differently than ER+ alone? I ask this because Ruth is 100% ER+/95% PR+ and so far has had a pretty dramatic response to pre-adjuvant TCH chemo.
2) Since Herceptin has been shown to work better with chemo is there some synergistic work between the two which indicates it either way for HER2+ or does that bring us again back to 1) above?
3) Would this data on ER+ testing mostly affect those who are ER-? (in the idea that they might need the hormone treament after all if test was in accurate) Would it possibly affect treatment regime decisions on those who are showing ER+?
4) Lani, can you translate this into a recommendation? When Ruth has her surgery and sentinel node biopsy should we ask for a different dye or since she's already established ER+ is it not a big deal? Can the dye affect anything else?
Lani, Your research is ALWaYS very interesting..tho hard for me to slog thru!  I always appreciate it when you put a few words in to translate! |

|

|
 07-10-2007, 09:18 AM
07-10-2007, 09:18 AM
|
#12
|
|
Senior Member
Join Date: Mar 2006
Posts: 4,778
|
wow--don't know if I have time to answer all but...
1) they have found that ER+PR+ react differently to antihormonals than ER+PR-. Hormonallly positive tumors receive much less benefit (if any from Chemo) her2+ER+ may be the exception, but it may not be all of them eg it may be those which are topoIIa + and respond to anthracyclines. It SEEMS her2+ER+ respond to taxanes, whether more or les than ER- is not known. It won't probably be known until they discover how to subclassify the different types of her2+ER+ bc and find which respond best to what treatment.
Ruth's dramatic response portends well!!!
2) did n't understand the question. Sorry!
3) If one is ER- and they miss it, treatment may not include antihormonals which may improve prognosis. If one is falsely diagnosed as ER+, perhaps one decided to forgo chemo thinking the benefit would be small and/or one would be treated with a drug which may have unnecessary side-effects
4) Don't know if the dye can affect anything else. Search for my post on the methylene blue and print it out. Other dyes were used in Europe, but cost more and may not be available to the surgeon here. I just read a paper showing that using the radioactive material alone can miss some metastatic deposits in sentinel nodes, hence the recommendation to use both.
If Ruth was ER+ when methylene blue was not used, the same tissue could be reexamined using another technique to reconfirm the ER positivity. A
pathologist at a breast cancer conference I attended two weekends ago brought up in front of all of his colleagues and oncologists that it is time to reexamine how ER is tested for as better, more reliable methods are available and/or testing in more than one way to assure the results are accurate may be in order.
I am certainly not an oncologist, pathologist, or breast surgeon or in any way qualified to advise you...just well-read.
If anyone is truly concerned, print out some of the articles or abstracts and take it with you when you ask questions. There are such things as pathologic second opinions where you don't even have to go, just send your slides. No
point in worrying unless it will change your treatment . One member of the board had tested initially ER+ on the needle biopsy, then ER- when the tumor was removed. Perhaps in that sort of instance suspicions might be raised. Remember mistakes are the exception, not the rule. But asking questions is part of the process...
Hope this helped more than raised worries!
|

|

|
 07-10-2007, 09:31 AM
07-10-2007, 09:31 AM
|
#13
|
|
Senior Member
Join Date: Mar 2006
Posts: 4,778
|
Flori
gotta go (long post for TSUND) so will make this fast
her2+ virtually always metastasizes at her2+ (can cite papers if needed)
Usually if discordant, method of her2 testing reexamined
At conferences I attend, including SABCS, the oncologists are always bemoaning the fact that they don't get a chance to get a biopsy of mets
(I raised my hand and asked why bone marrow biopsies aren't more prevalent to assess residual disease and was either ignored or scoffed at)
Lung mets can be dangerous to biopsy and sometimes very inaccessible.
ER negatives usually don't "turn positive" but giving lapatinib can cause a 40-70% increase in ERs I believe I read--so if you were .8% positive (considered negative) I suppose you could become positive.It isn't that the ER- tumors "turn" positive, it is that they were misdiagnosed for technical reasons initially or perhaps that a treatment allowed those cells which had ERs to be selected out (like weeds in a garden, if you use a weed killer that doesn't get dandelions, dandelions will no longer have to compete with the other weeds and will become predominant.
Walter Carney has published on the use of serum her2 to detect those tumors which start out her2- but become her2+ when they become resistant to antihormonals and/or recur.
Most answers in cancer are not "yes" and "no". It is far to complicated a field and too poorly understood. Let's hope it doesn't stay that way long!
|

|

|
 07-10-2007, 10:02 AM
07-10-2007, 10:02 AM
|
#14
|
|
Senior Member
Join Date: May 2007
Location: DFW area (TX)
Posts: 431
|
1) hmm...is the dye used on the intial needle biopsy as well as the sentinel node biopsy? (Ruth is getting "pre-surgery" chemo)
It has occured to me that in highly hormonal positive that the chemo may just be shutting down the ER via chemopause and this is the main way it works. HOWEVER I don't think that is true in Ruth's case as her dramatic response was very evident in 1st exam before 2nd treatment, and I've read that usually women have one more menstrual cycle before chemopause) Tho it's been 5 weeks and she's not had another cycle.
Lani, I was referring in #2 aboce to what I had read in reference to Herceptin working better WITH chemo than without, I believe regardless of ER status. So...this perhaps leads to the idea that all HER2+ should have chemo with Herceptin since we really need the Herceptin and you wnat it to work in the best possbile way? Or....it could translate into HER2+ tumors needing or responding to the chemo for some reason inherent in Her2+?
This has confused me, because I've read that herceptin works better with an intact immune system. So..since chemo generally can hurt the immune system, why does Herceptin work better with chemo???
THANKS~
Terri
THANK-yoU!
|

|

|
 07-10-2007, 11:56 AM
07-10-2007, 11:56 AM
|
#15
|
|
Senior Member
Join Date: Aug 2006
Posts: 3,380
|
Terri,
The benefit of using Herceptin in an uncompromised immune system is thought to be via "Natural Killer" (NK) cells. These cells are noticeably reduced following most chemo. I did read an abstract: http://www.ncbi.nlm.nih.gov/sites/en...&dopt=Abstract Titled "Paclitaxel probably enhances cytotoxicity of natural killer cells against breast carcinoma cells by increasing perforin production," which may explain the positive results seen when combining paclitaxel with Herceptin.
Hope this helps,
Hopeful |

|

|
 07-10-2007, 12:48 PM
07-10-2007, 12:48 PM
|
#16
|
|
Senior Member
Join Date: Nov 2004
Location: Misty woods of WA State
Posts: 4,128
|
 Tumor hormone chameleon
Tumor hormone chameleon
I know of two cases among my friends here who have had new mets change from hormone positive to negative. Therefore stopping the AI they had been on for years. Both are HER2 positive and disease was progressing.
In one case it was new nodes in the neck and another case was bone. Each had biopsies to see why the old treatments were no longer working.
Also heard discussion at San Antonio that new mets can change hormone status, but don't recall about ER/PR neg can become positive for certain.
(You would think I would have remembered that one since I am hormone neg!) Maybe the talk did not cover that case.
__________________
"When I hear music, I fear no danger. I am invulnerable. I see no foe. I am related to the earliest times, and to the latest." H.D. Thoreau
Live in the moment.
MY STORY SO FAR ~~~~
Found suspicious lump 9/2000
Lumpectomy, then node dissection and port placement
Stage IIB, 8 pos nodes of 18, Grade 3, ER & PR -
Adriamycin 12 weekly, taxotere 4 rounds
36 rads - very little burning
3 mos after rads liver full of tumors, Stage IV Jan 2002, one spot on sternum
Weekly Taxol, Navelbine, Herceptin for 27 rounds to NED!
2003 & 2004 no active disease - 3 weekly Herceptin + Zometa
Jan 2005 two mets to brain - Gamma Knife on Jan 18
All clear until treated cerebellum spot showing activity on Jan 2006 brain MRI & brain PET
Brain surgery on Feb 9, 2006 - no cancer, 100% radiation necrosis - tumor was still dying
Continue as NED while on Herceptin & quarterly Zometa
Fall-2006 - off Zometa - watching one small brain spot (scar?)
2007 - spot/scar in brain stable - finished anticoagulation therapy for clot along my port-a-catheter - 3 angioplasties to unblock vena cava
2008 - Brain and body still NED! Port removed and scans in Dec.
Dec 2008 - stop Herceptin - Vaccine Trial at U of W begun in Oct. of 2011
STILL NED everywhere in Feb 2014 - on wing & prayer
7/14 - Started twice yearly Zometa for my bones
Jan. 2015 checkup still shows NED
2015 Neuropathy in feet - otherwise all OK - still NED.
Same news for 2016 and all of 2017.
Nov of 2017 - had small skin cancer removed from my face. Will have Zometa end of Jan. 2018.
|

|

|
 07-10-2007, 04:01 PM
07-10-2007, 04:01 PM
|
#17
|
|
Senior Member
Join Date: Mar 2006
Posts: 4,778
|
There are many factors to consider
Probably not all ER+her2+ breast cancers are alike--perhaps some have topoIIa amplified, some not, and many other differences. When they are all lumped together the average of them do better with herceptin and chemo than with chemo alone and when ER+ tumors (both her2+--about 10% of them--and her2negative tumors, the other 90% of them) are considered as a whole on AVERAGE they hardly respond to chemo. This is probably because subsets are not identified and the results are skewed.
Perhaps there are a group of her2+ER+ tumors that would do fine with herceptin and fulvestrant (more likely than with herceptin and an AI, according to Dr. Slamon) without chemo, but all we can say now, is that when all her2+ tumors ER+ or not were lumped together in the HERA, NO American combined, TCH (BCRG009) and Fin Her trials that upon analysis of all lumped together the % improvement in recurrence and survival did not differ between those who were ER+ vs ER- ON AVERAGE.
Noone is an average. Someday, hopefully, each tumor will be evaluated individually and treated differently.
To more fully respond to your question--It seems a subgroup of her2+ tumors respond particularly well to anthracyclines (perhaps the TOPO IIa amplified group) and another group respond particularly well to herceptin and taxanes (perhaps the cMyc group and perhaps all subgroups, but we don't know for sure yet). They have not yet dissected this out with respect to ER+ vs ER- from the talks I have heard/papers I have read.
And no, if only a needle biopsy and not an open or excisional biopsy or node biopsy were done, methylene blue would not have been used, from what I understand.
|

|

|
 07-10-2007, 05:02 PM
07-10-2007, 05:02 PM
|
#18
|
|
Senior Member
Join Date: May 2007
Location: DFW area (TX)
Posts: 431
|
changing status of TOPO ll + HER2
Lani,
You are about 1000% ahead of me as far as being well read up,  but from what I understand about TOPO is that the latest info about Her2+ and TOPO flies in the face of what was thought previously. When Her2+ and Herceptin enters the equation, all of a sudden the anthrcycline is not necessary in chemo to achieve similar results (i.e the latest stats on TCH - San Antonio). The TOPO positive people do better in general, and that is true in HER2+ whether they do the AC+TH or TCH. I was given this answer when I asked our onc if the fact that Ruth was responding well to the TCH meant that she was TOPO negative as the other onc (not a bc specialist) had given us the hard core insistence that Ruth must have an anthracycline in case she was TOPO positive. I think I read something early on by Dr. Pegram (sp?) that indicates something similar. |

|

|
 07-10-2007, 05:05 PM
07-10-2007, 05:05 PM
|
#19
|
|
Senior Member
Join Date: May 2007
Location: DFW area (TX)
Posts: 431
|
Steph, were your friends that were progressing still on Herceptin?
And, do you know what percentage hormonally positve they were?
Thanks,
Terri
|

|

|
 07-10-2007, 05:26 PM
07-10-2007, 05:26 PM
|
#20
|
|
Senior Member
Join Date: May 2007
Location: DFW area (TX)
Posts: 431
|
addendum
...and I know this conflicts with the early indications of the Slamon trial. I think that the latest stats were different than the earlier ones.
Found what I read... and it was not Pegram, it was an interview with Dr. Burstein.
"DR BURSTEIN: The TOPO II issue, which has been discussed a lot since the BCIRG 006 data were presented in 2005, looks less relevant now with the 2006 data (Press 2005; Slamon 2006; [4.5, 4.6]).
The TOPO II gene is on human chromosome 17, not too far from the HER2/neu locus. In some cases of acquired HER2 gene amplification, you also have amplification of the TOPO II locus. TOPO II is a target of anthracyclines, and many people have suggested that TOPO II overexpression particularly identifies tumors that benefit from anthracyclines.
In the preliminary work from the BCIRG 006 trial that Dennis Slamon and Mike Press reported at the San Antonio meeting in 2005, they suggested that in TOPO II overexpressors, the anthracycline/ trastuzumab (AC 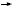 TH) arm was superior to the nonanthracycline/trastuzumab (TCH) arm. For the majority of tumors in which the TOPO II is not amplified, however, TCH was more or less equivalent to AC 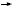 TH (Press 2005). If in the aggregate they’re the same, it washes out the effects of the TOPO II test question. I believe if clinicians decide that they can use a nonanthracycline/
trastuzumab-based regimen, it doesn’t matter whether they perform the TOPO II testing.
In the 35 percent of cases in which the tumor was both HER2-positive and TOPO II-positive, the curves all track similarly, which is a puzzle (Slamon 2006)" |

|

|
 Posting Rules
Posting Rules
|
You may not post new threads
You may not post replies
You may not post attachments
You may not edit your posts
HTML code is Off
|
|
|
All times are GMT -7. The time now is 12:58 PM.
|
